Calculating average atomic mass is a fundamental concept in chemistry, crucial for understanding the properties and behavior of elements. The average atomic mass of an element is a weighted average of the masses of its naturally occurring isotopes. In this article, we will delve into the world of average atomic mass calculations, exploring the concept, its importance, and providing a step-by-step guide on how to calculate it.
Key Points
- Understanding the concept of average atomic mass and its significance in chemistry
- Learning the formula for calculating average atomic mass
- Applying the formula to real-world examples
- Recognizing the importance of isotopic abundance in average atomic mass calculations
- Mastering the calculation of average atomic mass for elements with multiple isotopes
What is Average Atomic Mass?
Average atomic mass, also known as atomic weight, is the weighted average of the masses of the naturally occurring isotopes of an element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons, resulting in different masses. The average atomic mass is a crucial concept in chemistry, as it allows us to calculate the mass of a sample of an element, which is essential for various chemical reactions and calculations.
Importance of Average Atomic Mass
The average atomic mass is vital in chemistry, as it provides a way to calculate the mass of a sample of an element. This is essential for various chemical reactions, such as stoichiometry, where the ratio of reactants and products is critical. Additionally, the average atomic mass is used to calculate the molecular weight of compounds, which is essential for understanding their properties and behavior.
Calculating Average Atomic Mass
The formula for calculating average atomic mass is:
Average Atomic Mass = (Mass of Isotope 1 x Abundance of Isotope 1) + (Mass of Isotope 2 x Abundance of Isotope 2) +...
Where:
- Mass of Isotope 1, 2, etc. are the masses of the naturally occurring isotopes of the element
- Abundance of Isotope 1, 2, etc. are the percentages of each isotope in the naturally occurring mixture
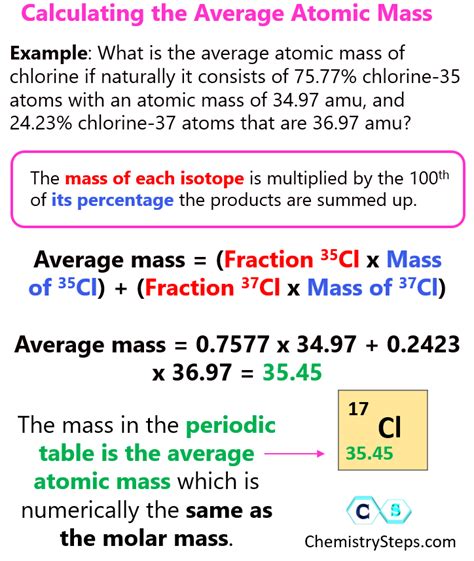
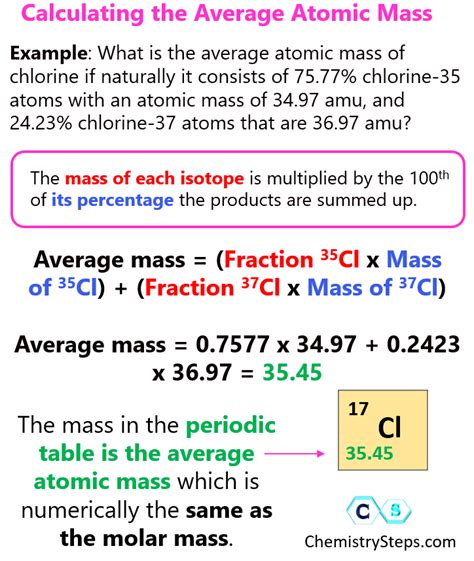
For example, let's calculate the average atomic mass of chlorine (Cl), which has two naturally occurring isotopes: Cl-35 and Cl-37.
| Isotope | Mass (u) | Abundance (%) |
|---|---|---|
| Cl-35 | 34.9689 | 75.78 |
| Cl-37 | 36.9659 | 24.22 |

Using the formula, we can calculate the average atomic mass of chlorine as follows:
Average Atomic Mass = (34.9689 x 0.7578) + (36.9659 x 0.2422)
Average Atomic Mass = 26.4963 + 8.9563
Average Atomic Mass = 35.4526 u
Isotopic Abundance
Isotopic abundance is critical in average atomic mass calculations, as it determines the contribution of each isotope to the overall mass. The abundance of an isotope is expressed as a percentage, and it can vary depending on the source and location of the element. For example, the abundance of Cl-35 and Cl-37 can vary slightly depending on the location and geological history of the sample.
Real-World Applications
Average atomic mass calculations have numerous real-world applications, including:
- Stoichiometry: calculating the mass of reactants and products in chemical reactions
- Molecular weight calculations: determining the mass of molecules and compounds
- Isotopic analysis: identifying the isotopic composition of samples and determining their origin
Conclusion
In conclusion, calculating average atomic mass is a fundamental concept in chemistry that requires a deep understanding of isotopes, their masses, and abundances. By applying the formula and considering the importance of isotopic abundance, chemists can accurately determine the average atomic mass of elements, which is essential for various chemical applications. Whether you’re a student or a professional, mastering average atomic mass calculations will enhance your understanding of chemistry and provide a solid foundation for further exploration and discovery.
What is the difference between atomic mass and average atomic mass?
+Atomic mass refers to the mass of a single atom, while average atomic mass is the weighted average of the masses of the naturally occurring isotopes of an element.
Why is isotopic abundance important in average atomic mass calculations?
+Isotopic abundance determines the contribution of each isotope to the overall mass, and it can vary depending on the source and location of the element.
What are some real-world applications of average atomic mass calculations?
+Average atomic mass calculations have numerous real-world applications, including stoichiometry, molecular weight calculations, and isotopic analysis.
Meta Description: Learn how to calculate average atomic mass with ease. Discover the importance of isotopic abundance and real-world applications of average atomic mass calculations. Master the formula and enhance your understanding of chemistry. (149 characters)