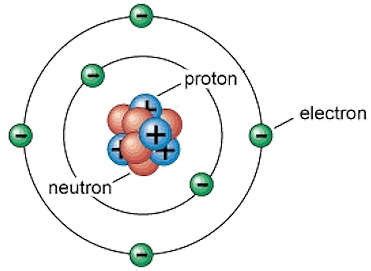
The Bohr atomic model, developed by Niels Bohr in 1913, revolutionized the understanding of atomic structure. This model posits that electrons occupy specific energy levels, or shells, around the nucleus of an atom. The energy levels are quantized, meaning that electrons can only occupy specific discrete levels, and not any energy level in between. The Bohr model was a significant improvement over earlier models, such as the Rutherford model, which proposed that electrons orbited the nucleus in a random manner.
In the context of carbon, the Bohr atomic model is particularly relevant. Carbon is the sixth element in the periodic table, with an atomic number of 6. This means that a neutral carbon atom has 6 protons in its nucleus and 6 electrons orbiting around it. According to the Bohr model, the 6 electrons in a carbon atom occupy the first two energy levels, or shells. The first energy level, also known as the 1s orbital, can hold up to 2 electrons, while the second energy level, or 2s and 2p orbitals, can hold up to 8 electrons. In the case of carbon, the first energy level is fully occupied by 2 electrons, and the remaining 4 electrons occupy the second energy level.
Key Points
- The Bohr atomic model proposes that electrons occupy specific energy levels, or shells, around the nucleus of an atom.
- Carbon is the sixth element in the periodic table, with an atomic number of 6.
- The 6 electrons in a carbon atom occupy the first two energy levels, or shells, according to the Bohr model.
- The first energy level is fully occupied by 2 electrons, and the remaining 4 electrons occupy the second energy level.
- The Bohr model was a significant improvement over earlier models, such as the Rutherford model.
Energy Levels and Electron Configuration

The energy levels, or shells, in the Bohr atomic model are labeled with the principal quantum number (n). The first energy level, or 1s orbital, has a principal quantum number of n = 1, while the second energy level, or 2s and 2p orbitals, has a principal quantum number of n = 2. The energy levels are further divided into subshells, which are labeled with the azimuthal quantum number (l). The 1s orbital has an azimuthal quantum number of l = 0, while the 2s orbital has an azimuthal quantum number of l = 0, and the 2p orbitals have an azimuthal quantum number of l = 1.
In the case of carbon, the electron configuration is 1s² 2s² 2p². This means that the first energy level is fully occupied by 2 electrons in the 1s orbital, and the second energy level is partially occupied by 4 electrons, with 2 electrons in the 2s orbital and 2 electrons in the 2p orbitals. The electron configuration of carbon is particularly important, as it determines the chemical properties of the element. The 4 electrons in the second energy level are available for bonding, which makes carbon a highly versatile element that can form a wide range of compounds.
Chemical Properties of Carbon
The chemical properties of carbon are largely determined by its electron configuration. The 4 electrons in the second energy level are available for bonding, which makes carbon a highly reactive element. Carbon can form covalent bonds with other atoms, such as hydrogen, oxygen, and nitrogen, to form a wide range of compounds. The ability of carbon to form long chains and rings of atoms, known as catenation, is particularly important, as it allows carbon to form complex molecules such as proteins, carbohydrates, and fats.
| Compound | Formula | Chemical Properties |
|---|---|---|
| Methane | CH₄ | Highly flammable, odorless gas |
| Carbon dioxide | CO₂ | Colorless, odorless gas, essential for plant growth |
| Glucose | C₆H₁₂O₆ | White, crystalline solid, primary source of energy for cells |

Quantum Mechanical Model

The Bohr atomic model, while a significant improvement over earlier models, has several limitations. The model does not account for the spin of electrons, which is a fundamental property of quantum mechanics. The model also does not account for the uncertainty principle, which states that it is impossible to know both the position and momentum of an electron with infinite precision. The quantum mechanical model, developed by Erwin Schrödinger and Werner Heisenberg, provides a more accurate description of the behavior of electrons in atoms.
In the quantum mechanical model, the electrons in an atom are described by a wave function, which gives the probability of finding an electron at a particular location. The wave function is obtained by solving the Schrödinger equation, which is a partial differential equation that describes the behavior of electrons in atoms. The solutions to the Schrödinger equation are known as atomic orbitals, which describe the distribution of electrons in an atom.
Atomic Orbitals
The atomic orbitals in the quantum mechanical model are labeled with the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (m). The 1s orbital, for example, has a principal quantum number of n = 1, an azimuthal quantum number of l = 0, and a magnetic quantum number of m = 0. The 2s orbital has a principal quantum number of n = 2, an azimuthal quantum number of l = 0, and a magnetic quantum number of m = 0. The 2p orbitals have a principal quantum number of n = 2, an azimuthal quantum number of l = 1, and magnetic quantum numbers of m = -1, 0, and 1.
In the case of carbon, the atomic orbitals are particularly important, as they determine the chemical properties of the element. The 1s orbital is fully occupied by 2 electrons, while the 2s and 2p orbitals are partially occupied by 4 electrons. The ability of carbon to form long chains and rings of atoms, known as catenation, is largely determined by the availability of electrons in the 2s and 2p orbitals.
What is the Bohr atomic model?
+The Bohr atomic model is a model of the atom that proposes that electrons occupy specific energy levels, or shells, around the nucleus of an atom.
What is the electron configuration of carbon?
+The electron configuration of carbon is 1s² 2s² 2p².
What are the chemical properties of carbon?
+The chemical properties of carbon are largely determined by its electron configuration. Carbon can form covalent bonds with other atoms, such as hydrogen, oxygen, and nitrogen, to form a wide range of compounds.
Meta Description: Learn about the Bohr atomic model and its application to carbon, including the electron configuration, chemical properties, and quantum mechanical model.