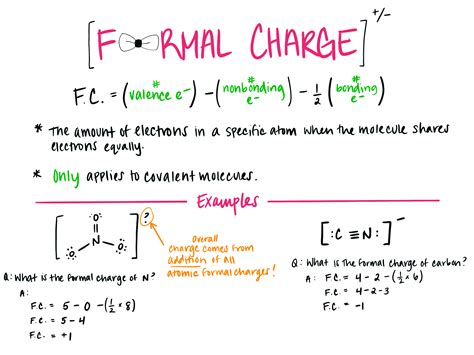
Calculating formal charge is a crucial step in understanding the structure and reactivity of molecules, particularly in organic and inorganic chemistry. The formal charge of an atom in a molecule is a measure of the difference between the number of electrons in an isolated atom and the number of electrons assigned to that atom in the molecule. Here, we will explore five ways to calculate formal charge, highlighting the importance of each method in different contexts.
Understanding Formal Charge
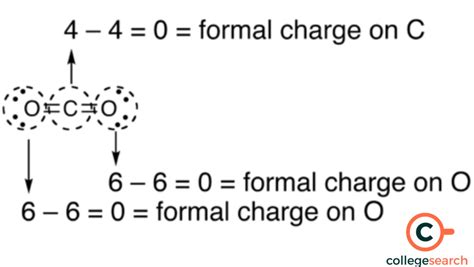
Before diving into the calculation methods, it’s essential to understand what formal charge represents. Formal charge is a bookkeeping method that helps chemists determine the most likely structure of a molecule based on the arrangement of electrons. It’s calculated by subtracting the number of non-bonding electrons and half of the bonding electrons from the total number of valence electrons of an atom in its ground state.
Method 1: Basic Formula Application
The basic formula for calculating formal charge (FC) is:
FC = V - (N + B/2)
Where:
- V = Number of valence electrons in the free atom
- N = Number of non-bonding electrons
- B = Number of bonding electrons
This method is straightforward and applies to most simple molecules. For example, calculating the formal charge of oxygen in water (H2O) involves recognizing that oxygen has 6 valence electrons, 4 of which are non-bonding (two lone pairs), and 4 are bonding (two single bonds with hydrogen, each bond representing 2 electrons shared between oxygen and hydrogen, hence 4 electrons).
| Atom | Valence Electrons (V) | Non-bonding Electrons (N) | Bonding Electrons (B) | Formal Charge (FC) |
|---|---|---|---|---|
| Oxygen in H2O | 6 | 4 | 4 | 0 |
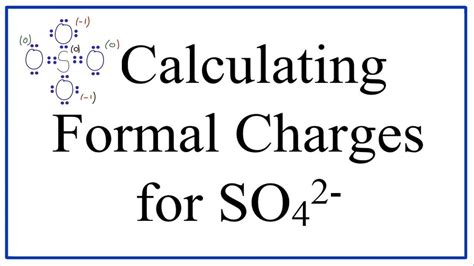
Advanced Calculation Methods
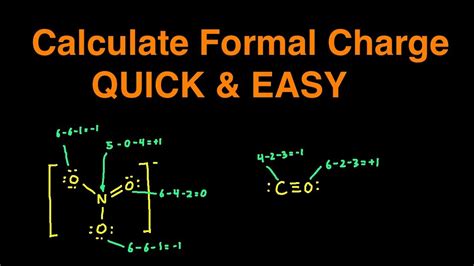
Beyond the basic formula, there are more nuanced approaches to calculating formal charge, especially in complex molecules or when dealing with resonance structures.
Method 2: Resonance Structures Analysis
For molecules that exhibit resonance, calculating formal charge involves analyzing each resonance structure. The structure with the lowest formal charges on atoms, especially on highly electronegative atoms like oxygen and nitrogen, is often considered the most stable or major contributor to the hybrid structure.
Method 3: Considering Electronegativity
Electronegativity plays a significant role in the distribution of electrons in a bond. When calculating formal charge, considering the electronegativity of atoms involved in a bond can provide a more realistic picture of electron distribution, especially in polar bonds.
Method 4: Utilizing Lewis Structures
Lewis structures are a visual representation of the valence electrons in a molecule. By drawing and analyzing Lewis structures, chemists can easily identify the number of non-bonding and bonding electrons around each atom, facilitating the calculation of formal charge.
Method 5: Computational Chemistry Tools
In modern chemistry, computational tools and software can calculate formal charges based on quantum mechanical calculations. These methods provide a highly accurate estimation of electron distribution and can be particularly useful for complex molecules where manual calculations become impractical.
Key Points
- The basic formula for calculating formal charge is FC = V - (N + B/2), where V is the number of valence electrons, N is the number of non-bonding electrons, and B is the number of bonding electrons.
- Resonance structures and electronegativity considerations can refine formal charge calculations, especially in complex molecules.
- Lewis structures provide a visual aid for identifying non-bonding and bonding electrons.
- Computational chemistry tools offer accurate calculations of formal charge based on quantum mechanics.
- Understanding formal charge is crucial for predicting molecular stability and reactivity.
In conclusion, calculating formal charge is a fundamental aspect of chemistry that helps in understanding the electronic structure of molecules. By mastering the various methods of calculating formal charge, chemists can better predict the stability, reactivity, and properties of molecules, which is essential for advancements in fields like organic synthesis, materials science, and drug discovery.
What is the purpose of calculating formal charge in chemistry?
+Calculating formal charge helps chemists understand the electronic structure of molecules, predict stability and reactivity, and identify the most likely structure of a molecule among possible resonance structures.
How does electronegativity affect the calculation of formal charge?
+Electronegativity influences the distribution of electrons in a bond. In polar bonds, the more electronegative atom pulls electrons closer, which can affect the calculation of formal charge by altering the perceived number of bonding electrons associated with each atom.
What are the limitations of manual formal charge calculations?
+Manual calculations can become complex and time-consuming, especially for large molecules. They also rely on assumptions about electron distribution, which may not always accurately reflect the molecule’s true electronic structure.