The ClF3 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of chlorine trifluoride. To understand this structure, it's essential to have a basic knowledge of chemistry, particularly in the areas of molecular bonding and Lewis structures. In this article, we will delve into the world of ClF3, exploring its Lewis structure, molecular geometry, and the underlying principles that govern its formation.
Key Points
- The ClF3 molecule consists of one chlorine atom and three fluorine atoms.
- The Lewis structure of ClF3 is drawn using the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons.
- The molecular geometry of ClF3 is T-shaped, resulting from the interaction of the lone pair and bonding pairs of electrons.
- Understanding the ClF3 Lewis structure is crucial for predicting its chemical properties and reactivity.
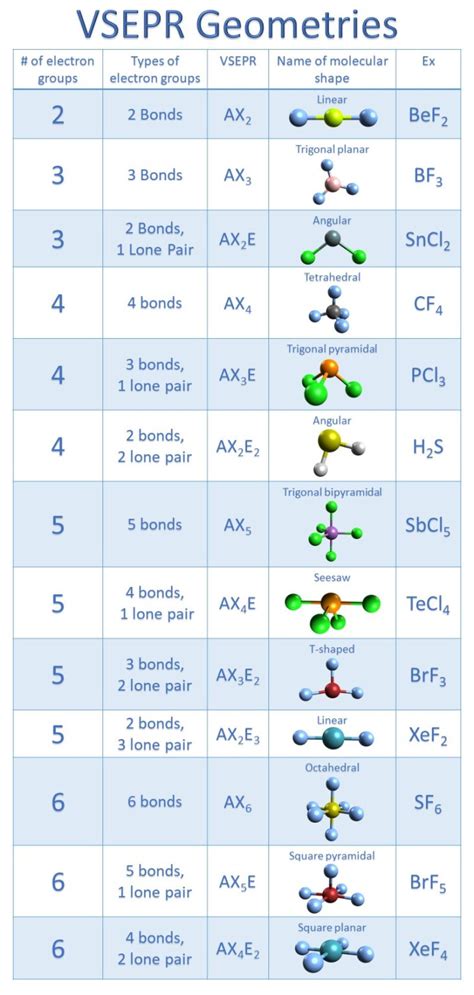
- The VSEPR theory is used to predict the molecular geometry of ClF3, taking into account the repulsions between electron pairs.
Introduction to ClF3 Lewis Structure
The ClF3 molecule is composed of one chlorine atom and three fluorine atoms. To draw the Lewis structure, we start by calculating the total number of valence electrons, which is 28 (7 from chlorine and 21 from the three fluorine atoms). We then use the octet rule to arrange the electrons around the atoms, ensuring that each atom has a full outer shell of eight electrons.
Drawing the ClF3 Lewis Structure
To draw the Lewis structure, we begin by placing the chlorine atom in the center and the three fluorine atoms around it. We then draw single bonds between the chlorine and each fluorine atom, which accounts for six electrons. The remaining 22 electrons are distributed around the atoms, with three lone pairs on each fluorine atom and two lone pairs on the chlorine atom. This arrangement satisfies the octet rule for all atoms, resulting in a stable molecule.
| Atom | Valence Electrons | Lone Pairs |
|---|---|---|
| Chlorine | 7 | 2 |
| Fluorine | 7 | 3 |
Molecular Geometry of ClF3
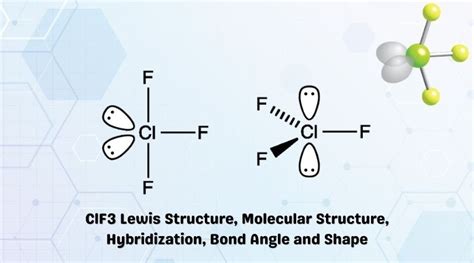
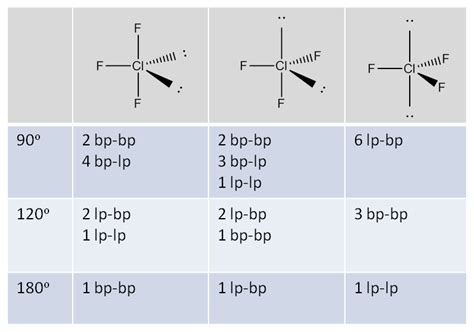
The molecular geometry of ClF3 is T-shaped, resulting from the interaction of the lone pair and bonding pairs of electrons. According to the VSEPR theory, the shape of a molecule is determined by the repulsions between electron pairs. In the case of ClF3, the two lone pairs on the chlorine atom occupy more space than the bonding pairs, resulting in a T-shaped geometry.
VSEPR Theory and ClF3
The VSEPR theory is used to predict the molecular geometry of ClF3, taking into account the repulsions between electron pairs. The theory states that electron pairs arrange themselves to minimize repulsions, resulting in a stable molecule. In the case of ClF3, the two lone pairs on the chlorine atom repel the bonding pairs, resulting in a T-shaped geometry.
The VSEPR theory is based on the following principles:
- Electron pairs repel each other.
- Lone pairs occupy more space than bonding pairs.
- The shape of a molecule is determined by the arrangement of electron pairs.
What is the molecular geometry of ClF3?
+The molecular geometry of ClF3 is T-shaped, resulting from the interaction of the lone pair and bonding pairs of electrons.
How is the ClF3 Lewis structure drawn?
+The ClF3 Lewis structure is drawn using the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons.
What is the VSEPR theory?
+The VSEPR theory is used to predict the molecular geometry of a molecule, taking into account the repulsions between electron pairs.
In conclusion, the ClF3 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of chlorine trifluoride. By understanding the Lewis structure and molecular geometry of ClF3, we can predict its chemical properties and reactivity. The VSEPR theory is a useful tool for predicting the molecular geometry of molecules, taking into account the repulsions between electron pairs. By applying these concepts, we can gain a deeper understanding of the chemical properties and behavior of molecules like ClF3.



