The density of water is a fundamental physical property that plays a crucial role in various scientific and engineering applications. At its core, density is defined as the mass of a substance per unit volume, typically expressed in units of grams per milliliter (g/mL) or kilograms per liter (kg/L). The density of water is approximately 1 gram per milliliter (g/mL) at standard temperature and pressure (STP) conditions, which are defined as 20°C (68°F) and 1 atmosphere (atm) of pressure.
Temperature Dependence of Water Density
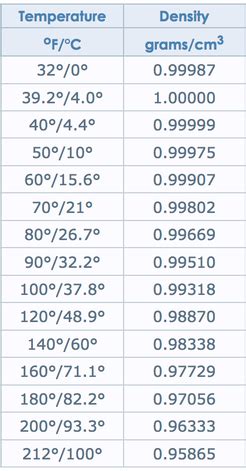
The density of water is not constant and varies with temperature. This variation is due to the thermal expansion of water molecules, which causes the molecules to move further apart as temperature increases, resulting in a decrease in density. Conversely, as temperature decreases, the molecules move closer together, increasing the density. The temperature dependence of water density is a critical factor in many applications, including engineering, chemistry, and biology. For instance, in aquatic ecosystems, changes in water temperature can significantly impact the density of water, affecting the distribution and behavior of aquatic organisms.
Density of Water at Different Temperatures
The density of water at various temperatures is as follows:
| Temperature (°C) | Density (g/mL) |
|---|---|
| 0 | 0.9998 |
| 10 | 0.9997 |
| 20 | 0.9982 |
| 30 | 0.9956 |
| 40 | 0.9922 |
| 50 | 0.9880 |
| 60 | 0.9832 |
| 70 | 0.9778 |
| 80 | 0.9718 |
| 90 | 0.9653 |
| 100 | 0.9584 |

As illustrated in the table, the density of water decreases with increasing temperature, with the most significant changes occurring between 0°C and 40°C.
Key Points
- The density of water is approximately 1 gram per milliliter (g/mL) at standard temperature and pressure (STP) conditions.
- The density of water varies with temperature, decreasing as temperature increases.
- The temperature dependence of water density is critical in various scientific and engineering applications.
- The density of water at 0°C is 0.9998 g/mL, while at 100°C it is 0.9584 g/mL.
- Understanding the density of water is essential for accurately calculating volumes and masses in chemical reactions and industrial processes.
Applications of Water Density

The density of water has numerous practical applications across various fields, including chemistry, biology, engineering, and environmental science. For example, in chemistry, the density of water is used to calculate the concentration of solutions and to determine the volume of reactants and products in chemical reactions. In biology, the density of water is crucial for understanding the behavior and distribution of aquatic organisms, as well as the transport of nutrients and pollutants in aquatic ecosystems.
Calculating Volumes and Masses
In many industrial and laboratory settings, accurate calculations of volumes and masses are critical for process optimization, quality control, and safety. The density of water is a key factor in these calculations, as it allows for the conversion between volume and mass. For instance, if a chemist needs to prepare a 1-liter solution with a specific concentration of a solute, knowing the density of water is essential for calculating the exact mass of the solute required.
Measurement of Water Density
There are several methods for measuring the density of water, including hydrometry, pycnometry, and vibrational spectroscopy. Each method has its advantages and limitations, and the choice of method depends on the specific application, required accuracy, and available resources. For instance, hydrometry involves measuring the volume of a known mass of water, while pycnometry involves measuring the mass of a known volume of water.
Hydrometry and Pycnometry
Hydrometry and pycnometry are two common methods for measuring the density of water. Hydrometry involves using a hydrometer, a device that measures the density of a liquid based on its buoyancy. Pycnometry, on the other hand, involves using a pycnometer, a device that measures the volume of a known mass of a substance. Both methods provide accurate measurements of water density but require careful calibration and attention to detail to ensure reliable results.
What is the density of water at 25°C?
+The density of water at 25°C is approximately 0.9970 g/mL.
How does temperature affect the density of water?
+Temperature affects the density of water by causing the molecules to move further apart as temperature increases, resulting in a decrease in density, and moving closer together as temperature decreases, resulting in an increase in density.
What are some common applications of water density?
+Some common applications of water density include calculating volumes and masses in chemical reactions, understanding the behavior and distribution of aquatic organisms, and determining the concentration of solutions.
In conclusion, the density of water is a fundamental physical property that plays a crucial role in various scientific and engineering applications. Understanding the temperature dependence of water density and its practical applications is essential for ensuring accuracy and precision in many fields. By recognizing the importance of water density and its measurement, scientists and engineers can optimize processes, improve safety, and advance our knowledge of the natural world.