The concept of electron charge is fundamental to understanding the behavior of matter at the atomic and subatomic level. Electron charge refers to the inherent property of electrons to carry a negative electric charge. This charge is a cornerstone of electromagnetism and plays a crucial role in the structure of atoms, molecules, and solids. Here are five key facts about electron charge that highlight its significance and characteristics:
Key Points
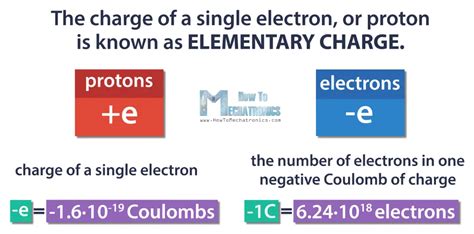
- The charge of an electron is a fundamental constant of nature, denoted as e and measured to be approximately 1.60217662 × 10^−19 coulombs.
- Electrons are negatively charged, which means they are attracted to positively charged particles, such as protons, and repelled by other negatively charged particles.
- The charge of an electron is quantized, meaning it comes in discrete packets (quanta) rather than being continuous. This quantization is a key feature of quantum mechanics.
- Electron charge is responsible for the chemical properties of elements. The number of electrons in an atom's outermost shell determines its reactivity and the types of chemical bonds it can form.
- Understanding electron charge is essential for the development of electronic devices. The flow of electrons (electric current) is the basis for all electronic circuits, from simple devices like flashlights to complex systems like computers.
Electron Charge and Atomic Structure

In the context of atomic structure, electron charge is critical. Atoms are composed of a nucleus, which contains protons (positively charged) and neutrons (neutral), surrounded by electrons. The number of electrons in a neutral atom equals the number of protons in the nucleus, balancing the positive and negative charges. The arrangement of electrons in different energy levels or shells around the nucleus determines the chemical properties of an element. Electron charge and its distribution are key factors in the formation of chemical bonds between atoms, whether ionic, covalent, or metallic.
Quantization of Electron Charge
The concept of quantized electron charge is a fundamental aspect of quantum mechanics. Unlike classical physics, where physical quantities like energy and charge can vary continuously, quantum mechanics introduces a granular or discrete nature to these quantities. The charge of an electron is a basic unit of charge, and all free charges are whole-number multiples of this unit. This quantization has profound implications for understanding phenomena at the atomic and subatomic level, including the behavior of electrons in solids, which underpins the operation of electronic devices.
| Property | Value |
|---|---|
| Charge of an Electron | 1.60217662 × 10^−19 C |
| Mass of an Electron | 9.10938356 × 10^−31 kg |
| Spin of an Electron | 1/2 |

Electron Charge in Electronic Devices

The flow of electrons, or electric current, is the basis of all electronic devices. Understanding electron charge is crucial for designing and operating electronic circuits. The movement of electrons through a conductor, such as a wire, constitutes an electric current. Electronic devices, from simple resistors and capacitors to complex integrated circuits, rely on the controlled flow of electrons to perform their functions. The manipulation of electron charge is also at the heart of semiconductor technology, which underpins modern computing and communication systems.
Chemical Properties and Electron Charge
The chemical properties of elements are directly related to their electron configuration, particularly the arrangement of electrons in the outermost shell. The number of electrons in this shell determines the valence of an element and its tendency to form ions or share electrons in covalent bonds. Understanding electron charge and its role in chemical bonding is essential for predicting the reactivity of substances and designing new materials with specific properties. This knowledge has applications in fields such as materials science, chemistry, and pharmacology.
What is the significance of electron charge in chemistry?
+Electron charge plays a crucial role in determining the chemical properties of elements. It influences the formation of chemical bonds and the reactivity of substances.
How is electron charge quantized?
+Electron charge is quantized because it comes in discrete packets (quanta) rather than being continuous. This is a fundamental principle of quantum mechanics.
What role does electron charge play in electronic devices?
+Electron charge is the basis of electric current, which is essential for the operation of all electronic devices. The controlled flow of electrons enables devices to perform their functions.
In conclusion, electron charge is a fundamental property that underlies many aspects of physics, chemistry, and engineering. Its quantized nature, role in chemical bonding, and significance in electronic devices make it a critical concept for understanding and developing modern technologies. As research continues to refine our understanding of electron charge and its applications, we can expect advancements in fields such as materials science, electronics, and energy storage, ultimately shaping the future of technology and society.