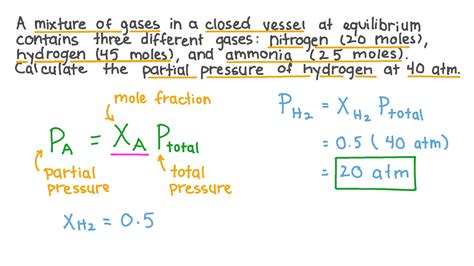
Calculating partial pressure is a crucial concept in chemistry and physics, particularly in the study of gases. Partial pressure refers to the pressure exerted by a single component of a mixture of gases. It is a fundamental principle in understanding the behavior of gases and is widely applied in various fields, including chemistry, physics, and engineering. In this article, we will explore five ways to calculate partial pressure, highlighting the underlying principles, formulas, and practical applications.
Understanding Partial Pressure
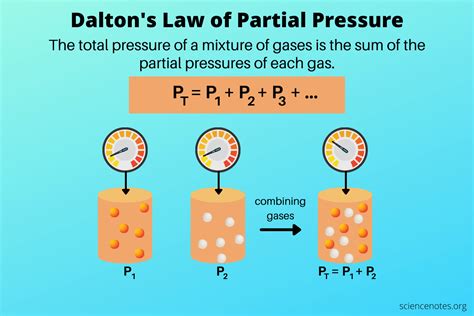
Before diving into the calculation methods, it is essential to understand the concept of partial pressure. According to Dalton’s Law of Partial Pressures, the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas in the mixture. The partial pressure of a gas is proportional to its mole fraction in the mixture and the total pressure of the system. This principle is crucial in calculating the partial pressure of a specific gas in a mixture.
Dalton’s Law of Partial Pressures
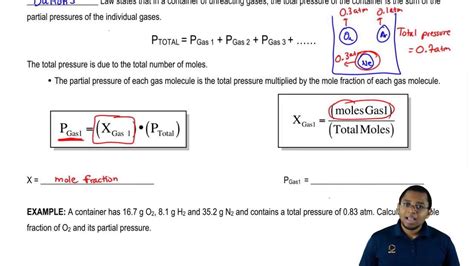
Dalton’s Law states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. Mathematically, this can be expressed as: P_total = P_1 + P_2 +… + P_n, where P_total is the total pressure of the mixture, and P_1, P_2,…, P_n are the partial pressures of each gas. This law provides the foundation for calculating partial pressure using various methods.
Key Points
- Partial pressure is the pressure exerted by a single component of a mixture of gases.
- Dalton's Law of Partial Pressures states that the total pressure of a mixture is equal to the sum of the partial pressures of each gas.
- The partial pressure of a gas is proportional to its mole fraction in the mixture and the total pressure of the system.
- Calculating partial pressure is crucial in understanding the behavior of gases in various fields, including chemistry, physics, and engineering.
- There are multiple methods to calculate partial pressure, each with its underlying principles and applications.
Method 1: Using Dalton’s Law
The first method to calculate partial pressure is by using Dalton’s Law directly. If the total pressure of the mixture and the mole fractions of the gases are known, the partial pressure of each gas can be calculated. The formula for calculating partial pressure using Dalton’s Law is: P_i = x_i * P_total, where P_i is the partial pressure of gas i, x_i is the mole fraction of gas i, and P_total is the total pressure of the mixture.
Example Calculation
For example, consider a mixture of oxygen (O2) and nitrogen (N2) at a total pressure of 1 atm. If the mole fraction of O2 is 0.2 and the mole fraction of N2 is 0.8, the partial pressure of O2 can be calculated as: P_O2 = 0.2 * 1 atm = 0.2 atm. Similarly, the partial pressure of N2 can be calculated as: P_N2 = 0.8 * 1 atm = 0.8 atm.
| Gas | Mole Fraction | Partial Pressure (atm) |
|---|---|---|
| O2 | 0.2 | 0.2 |
| N2 | 0.8 | 0.8 |
Method 2: Using the Ideal Gas Law
The second method to calculate partial pressure is by using the ideal gas law. The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. By rearranging the ideal gas law, the partial pressure of a gas can be calculated as: P_i = n_i * R * T / V, where P_i is the partial pressure of gas i, n_i is the number of moles of gas i, R is the gas constant, T is the temperature, and V is the volume.
Example Calculation
For example, consider a mixture of carbon dioxide (CO2) and helium (He) at a temperature of 298 K and a volume of 10 L. If the number of moles of CO2 is 0.5 mol and the number of moles of He is 1.5 mol, the partial pressure of CO2 can be calculated as: P_CO2 = 0.5 mol * 0.0821 L atm/mol K * 298 K / 10 L = 1.22 atm. Similarly, the partial pressure of He can be calculated as: P_He = 1.5 mol * 0.0821 L atm/mol K * 298 K / 10 L = 3.67 atm.
Method 3: Using Henry’s Law
The third method to calculate partial pressure is by using Henry’s Law, which applies to the solubility of gases in liquids. Henry’s Law states that the partial pressure of a gas is directly proportional to its concentration in the liquid. Mathematically, this can be expressed as: P_i = k * c_i, where P_i is the partial pressure of gas i, k is the Henry’s Law constant, and c_i is the concentration of gas i in the liquid.
Example Calculation
For example, consider a solution of oxygen (O2) in water at a temperature of 298 K. If the concentration of O2 in the water is 0.01 M and the Henry’s Law constant for O2 is 0.0267 L atm/mol, the partial pressure of O2 can be calculated as: P_O2 = 0.0267 L atm/mol * 0.01 M = 0.000267 atm.
Method 4: Using the Partial Pressure Formula

The fourth method to calculate partial pressure is by using the partial pressure formula, which is derived from Dalton’s Law. The formula is: P_i = (n_i / n_total) * P_total, where P_i is the partial pressure of gas i, n_i is the number of moles of gas i, n_total is the total number of moles, and P_total is the total pressure.
Example Calculation
For example, consider a mixture of nitrogen (N2) and oxygen (O2) at a total pressure of 1 atm. If the number of moles of N2 is 2 mol and the number of moles of O2 is 1 mol, the partial pressure of N2 can be calculated as: P_N2 = (2 mol / 3 mol) * 1 atm = 0.67 atm. Similarly, the partial pressure of O2 can be calculated as: P_O2 = (1 mol / 3 mol) * 1 atm = 0.33 atm.
Method 5: Using Experimental Data
The fifth method to calculate partial pressure is by using experimental data. This method involves measuring the total pressure and the composition of the gas mixture using techniques such as gas chromatography or mass spectrometry. The partial pressure of each gas can then be calculated using Dalton’s Law or the ideal gas law.
Example Calculation
For example, consider a gas mixture consisting of methane (CH4), ethane (C2H6), and propane (C3H8). If the total pressure is measured to be 2 atm and the composition of the mixture is found to be 50% CH4, 30% C2H6, and 20% C3H8, the partial pressure of each gas can be calculated using Dalton’s Law. The partial pressure of CH4 would be: P_CH4 = 0.5 * 2 atm = 1 atm. Similarly, the partial pressure of C2H6 would be: P_C2H6 = 0.3 * 2 atm = 0.6 atm, and the partial pressure of C3H8 would be: P_C3H8 = 0.2 * 2 atm = 0.4 atm.
What is the difference between partial pressure and total pressure?
+Partial pressure is the pressure exerted by a single component of a mixture of gases, while total pressure is the sum of the partial pressures of all the gases in the mixture.
How do you calculate partial pressure using Dalton's Law?
+Dalton's Law states that the total pressure of a mixture is equal to the sum of the partial pressures of each gas. The partial pressure of each gas can be calculated as: P_i = x_i \* P_total, where P_i is the partial pressure of gas i, x_i is the mole fraction of gas i, and P_total is the total pressure.
What is the ideal gas law, and how is it used to calculate partial pressure?
+The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. The partial pressure of a gas can be calculated using the ideal gas law by rearranging the equation to: P_i = n_i \* R \* T / V, where P_i is the partial pressure of gas i, n_i is the number of moles of gas i, R is the gas constant, T is the temperature, and V is the volume.
In conclusion, calculating partial pressure is a fundamental concept in understanding the behavior of gases. The five methods discussed in this article, including using Dalton’s Law, the ideal gas law, Henry’s Law, the partial pressure formula, and experimental data, provide a comprehensive approach to calculating partial pressure. By understanding these methods and their applications, individuals can better appreciate the complexities of gas mixtures and the importance of partial pressure in various fields.