Electrons, the tiny negatively charged particles that orbit the nucleus of an atom, play a crucial role in the structure and properties of matter. Finding electrons is essential in various fields, including physics, chemistry, and materials science. In this article, we will explore five ways to find electrons, discussing the theoretical foundations, practical applications, and limitations of each method.
Key Points
- Understanding the electron configuration of atoms is crucial for finding electrons
- Scanning Tunneling Microscopy (STM) can visualize electrons on the surface of materials
- Electron Spin Resonance (ESR) spectroscopy detects unpaired electrons in molecules
- X-ray Photoelectron Spectroscopy (XPS) measures the binding energy of electrons in atoms
- Quantum Mechanical Calculations can predict the behavior of electrons in complex systems
Understanding Electron Configuration

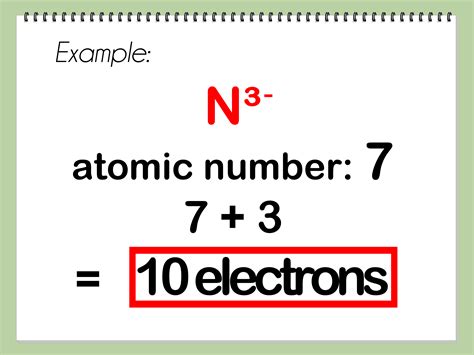
To find electrons, it is essential to understand the electron configuration of atoms. Electron configuration refers to the arrangement of electrons in an atom, which determines the chemical properties of an element. The electron configuration can be described using the Aufbau principle, the Pauli exclusion principle, and Hund’s rule. By applying these principles, scientists can predict the energy levels and orbital occupancies of electrons in an atom.
Scanning Tunneling Microscopy (STM)
Scanning Tunneling Microscopy (STM) is a technique that allows scientists to visualize electrons on the surface of materials. STM works by scanning a sharp tip over the surface of a material, detecting the tunneling current between the tip and the surface. This current is proportional to the density of states of the electrons on the surface, providing a detailed image of the electron distribution. STM has been instrumental in studying the surface properties of materials, including the behavior of electrons in nanoscale systems.
| Technique | Description | Advantages |
|---|---|---|
| Scanning Tunneling Microscopy (STM) | Visualizes electrons on the surface of materials | High spatial resolution, sensitive to surface electronic structure |
| Electron Spin Resonance (ESR) Spectroscopy | Detects unpaired electrons in molecules | High sensitivity, provides information on electron spin dynamics |
| X-ray Photoelectron Spectroscopy (XPS) | Measures the binding energy of electrons in atoms | Element-specific, provides information on chemical bonding and oxidation states |

Electron Spin Resonance (ESR) Spectroscopy

Electron Spin Resonance (ESR) spectroscopy is a technique that detects unpaired electrons in molecules. ESR works by applying a magnetic field to a sample, which causes the unpaired electrons to resonate at a specific frequency. By measuring the resonance frequency and intensity, scientists can gain information on the electron spin dynamics and the local environment of the unpaired electrons. ESR spectroscopy has been widely used in studying the properties of free radicals, transition metal complexes, and biological systems.
X-ray Photoelectron Spectroscopy (XPS)
X-ray Photoelectron Spectroscopy (XPS) is a technique that measures the binding energy of electrons in atoms. XPS works by irradiating a sample with X-rays, which causes the electrons to be emitted from the surface. By measuring the kinetic energy of the emitted electrons, scientists can determine the binding energy of the electrons in the atom. XPS provides element-specific information on the chemical bonding and oxidation states of atoms, making it a powerful tool for studying the surface properties of materials.
Quantum Mechanical Calculations
Quantum Mechanical Calculations are a powerful tool for predicting the behavior of electrons in complex systems. By solving the Schrödinger equation, scientists can calculate the electronic structure and properties of molecules and materials. Quantum Mechanical Calculations have been widely used in studying the properties of molecules, solids, and nanoscale systems, providing valuable insights into the behavior of electrons in these systems.
What is the importance of understanding electron configuration?
+Understanding electron configuration is crucial for predicting the chemical properties of elements and the behavior of electrons in atoms and molecules.
What is the difference between STM and ESR spectroscopy?
+STM visualizes electrons on the surface of materials, while ESR spectroscopy detects unpaired electrons in molecules.
What is the advantage of using XPS?
+XPS provides element-specific information on the chemical bonding and oxidation states of atoms, making it a powerful tool for studying the surface properties of materials.
In conclusion, finding electrons is a complex task that requires a deep understanding of the electron configuration of atoms and the techniques used to detect and visualize electrons. By applying the principles of electron configuration and the techniques of STM, ESR spectroscopy, XPS, and Quantum Mechanical Calculations, scientists can gain valuable insights into the behavior of electrons in complex systems, advancing our knowledge of materials science and chemistry.