Light is a fundamental component of our universe, and its influence on chemical reactions is a subject of ongoing research and fascination. The energy from light can initiate, accelerate, or modify chemical reactions, a phenomenon known as photochemistry. This field has numerous applications, ranging from the synthesis of complex molecules to the development of new materials and technologies. In this article, we will delve into five ways light drives reactions, exploring the principles, mechanisms, and examples of photochemical processes.
Key Points
- Photoexcitation of molecules leading to reaction initiation
- Role of light in photosynthesis and its applications
- Photocatalysis for environmental remediation and energy production
- Light-driven synthesis of complex organic molecules
- Application of photochemistry in biomedical fields
Photoexcitation and Reaction Initiation

The absorption of light by molecules can lead to their excitation, a state where the molecule has excess energy. This excess energy can be utilized to overcome the activation barrier of a reaction, thus initiating a chemical transformation. The process of photoexcitation is fundamental to understanding how light drives reactions. For instance, in the presence of light, certain molecules can undergo isomerization, where the light energy facilitates the rearrangement of molecular structure. This principle is crucial in various photochemical reactions, including those involved in the synthesis of vitamins and the degradation of pollutants.
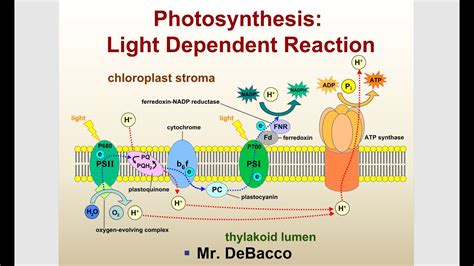
Photosynthesis and Its Applications
One of the most significant examples of light driving reactions is photosynthesis, the process by which plants, algae, and certain bacteria convert light energy into chemical energy. This complex process involves the absorption of light by pigments such as chlorophyll, leading to the production of ATP and NADPH, which are then used to convert CO2 into organic compounds like glucose. Understanding and mimicking photosynthesis has inspired the development of artificial photosynthetic systems aimed at producing biofuels, hydrogen, and other valuable chemicals. Moreover, insights from photosynthesis have guided the creation of more efficient solar cells and photocatalytic systems for water splitting and pollution remediation.
Photocatalysis for Environmental Remediation

Photocatalysis refers to the acceleration of a chemical reaction in the presence of light and a catalyst. This process has been extensively explored for environmental applications, including the degradation of organic pollutants in water and air. Titanium dioxide (TiO2) is a commonly used photocatalyst due to its ability to absorb UV light, generating reactive species that can oxidize and break down pollutants. Photocatalytic systems have been designed for the treatment of wastewater, the removal of volatile organic compounds from indoor air, and even for self-cleaning surfaces. The efficiency and selectivity of photocatalytic reactions can be tuned by modifying the catalyst’s surface, using different light sources, or combining photocatalysis with other treatment technologies.
Light-Driven Synthesis of Complex Molecules
Light can be a valuable tool in organic synthesis, offering a means to initiate reactions under mild conditions, which can be beneficial for the synthesis of complex and sensitive molecules. Photochemical reactions can be highly selective, allowing for the formation of specific products that might be challenging to obtain through traditional thermal reactions. For example, photoinduced electron transfer reactions can be used to generate radicals, which can then participate in various bond-forming reactions. This approach has been applied in the synthesis of natural products, pharmaceuticals, and materials science, demonstrating the versatility of photochemistry in modern synthesis.
Applications in Biomedical Fields
The application of light in driving reactions has significant implications in biomedical fields, particularly in photodynamic therapy (PDT), where light is used to kill cancer cells or bacteria. In PDT, a photosensitizer is first administered to the target area; upon irradiation with light of a specific wavelength, the photosensitizer produces reactive oxygen species that can destroy the targeted cells. This method has been approved for the treatment of certain types of cancer and is under investigation for its potential in combating antibiotic-resistant bacteria. Furthermore, photochemistry plays a role in the development of new diagnostic tools, such as fluorescence imaging, which relies on the emission of light by fluorescent molecules to visualize biological processes at the molecular level.
| Application | Description |
|---|---|
| Photosynthesis | Conversion of light energy into chemical energy |
| Photocatalysis | Acceleration of chemical reactions by light |
| Organic Synthesis | Use of light to initiate and control chemical reactions |
| Photodynamic Therapy | Light-induced production of reactive species for therapeutic applications |
| Fluorescence Imaging | Visualization of biological processes using fluorescence |

In conclusion, the role of light in driving chemical reactions is multifaceted and profound, influencing fields from environmental science to medicine. As research continues to uncover the intricacies of photochemical processes, we can expect the development of novel technologies and applications that leverage the power of light to initiate, accelerate, and control chemical transformations. The future of photochemistry holds much promise, with potential breakthroughs in energy production, pollution remediation, and the synthesis of complex molecules, underscoring the importance of continued exploration and innovation in this dynamic field.
What is the basic principle behind photochemistry?
+Photochemistry is based on the principle that light energy can be absorbed by molecules, leading to their excitation and subsequent participation in chemical reactions.
How does photocatalysis contribute to environmental remediation?
+Photocatalysis uses light to activate catalysts, which then generate reactive species that can degrade pollutants in water and air, offering a powerful tool for environmental cleanup.
What are the potential biomedical applications of photochemistry?
+Photochemistry has several biomedical applications, including photodynamic therapy for cancer treatment, fluorescence imaging for diagnostics, and the development of new therapeutic agents activated by light.