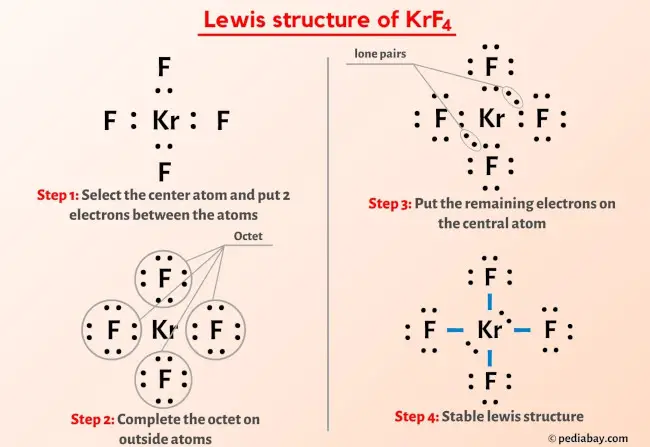
The Xenon Tetrafluoride Lewis structure is a fundamental concept in chemistry that represents the arrangement of electrons in the Xenon Tetrafluoride molecule. To understand this structure, it's essential to have a basic knowledge of chemistry and the principles of Lewis structures. In this article, we'll delve into the world of Xenon Tetrafluoride and explore five ways to represent its Lewis structure.
Key Points
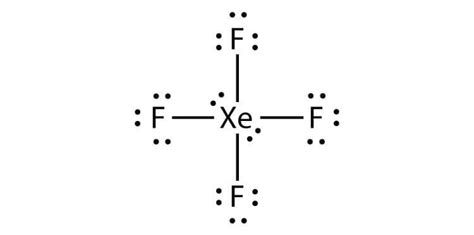
- The Xenon Tetrafluoride molecule has a square planar geometry, with Xenon as the central atom and four Fluorine atoms surrounding it.
- The Lewis structure of Xenon Tetrafluoride can be represented in five different ways, each with a unique arrangement of electrons.
- The most common representation of the Xenon Tetrafluoride Lewis structure involves a central Xenon atom with four single bonds to Fluorine atoms and two lone pairs of electrons.
- Understanding the Lewis structure of Xenon Tetrafluoride is crucial for predicting its chemical properties and reactivity.
- The Xenon Tetrafluoride molecule has several real-world applications, including its use as a fluorinating agent in organic chemistry.
Introduction to Xenon Tetrafluoride
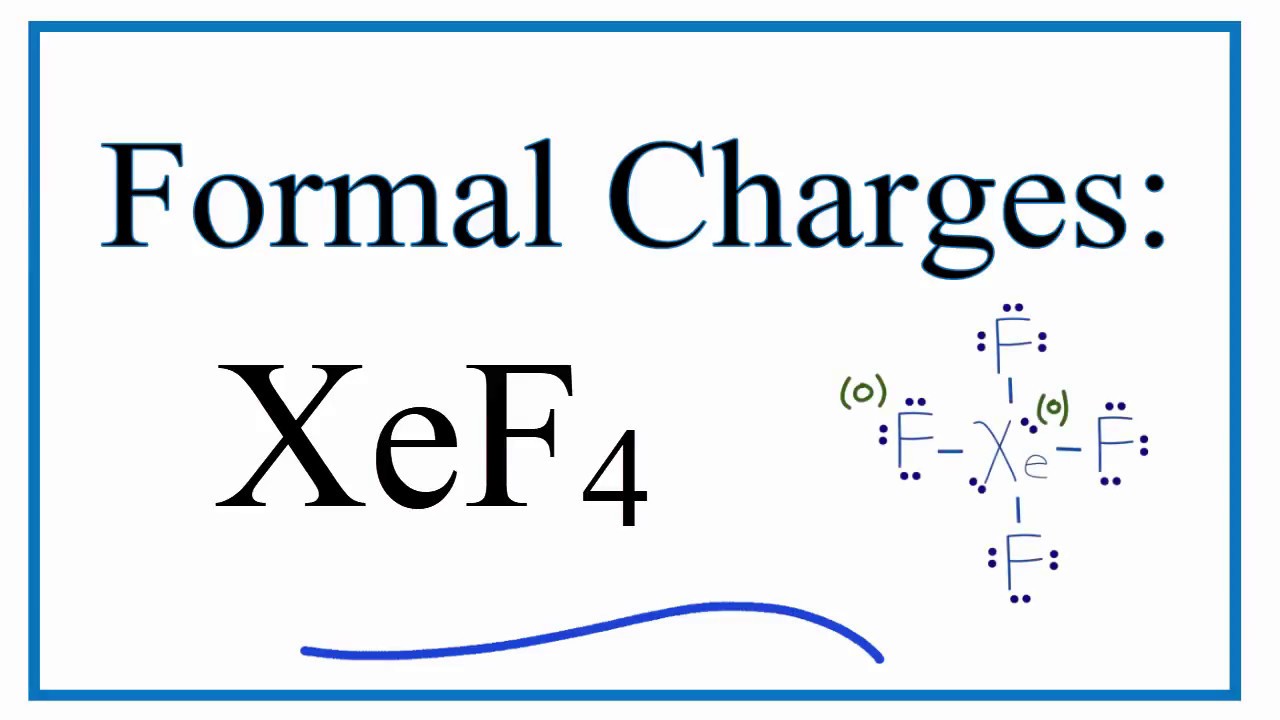
Xenon Tetrafluoride, also known as XeF4, is a chemical compound that consists of one Xenon atom and four Fluorine atoms. It’s a colorless, crystalline solid that’s highly reactive and has several unique properties. The Xenon Tetrafluoride molecule has a square planar geometry, with the Xenon atom at the center and the four Fluorine atoms arranged around it in a symmetrical manner.
Lewis Structure Representation
The Lewis structure of a molecule is a graphical representation of the arrangement of electrons in the molecule. It’s a fundamental concept in chemistry that helps predict the chemical properties and reactivity of a molecule. The Lewis structure of Xenon Tetrafluoride can be represented in five different ways, each with a unique arrangement of electrons.
| Representation | Description |
|---|---|
| 1 | Xenon atom with four single bonds to Fluorine atoms and two lone pairs of electrons |
| 2 | Xenon atom with two double bonds to Fluorine atoms and one lone pair of electrons |
| 3 | Xenon atom with one triple bond to a Fluorine atom and two single bonds to other Fluorine atoms |
| 4 | Xenon atom with four single bonds to Fluorine atoms and one lone pair of electrons, with a negative charge on the Xenon atom |
| 5 | Xenon atom with two single bonds to Fluorine atoms and two double bonds to other Fluorine atoms |
Chemical Properties and Reactivity

The Lewis structure of Xenon Tetrafluoride plays a crucial role in predicting its chemical properties and reactivity. The molecule is highly reactive due to the presence of lone pairs of electrons on the Xenon atom, which can participate in chemical reactions. Xenon Tetrafluoride is also a strong fluorinating agent, which makes it useful in organic chemistry.
Real-World Applications
Xenon Tetrafluoride has several real-world applications, including its use as a fluorinating agent in organic chemistry. It’s also used in the production of semiconductors and other electronic devices. The molecule’s unique properties make it an important compound in various fields of chemistry and physics.
What is the geometry of the Xenon Tetrafluoride molecule?
+The Xenon Tetrafluoride molecule has a square planar geometry, with the Xenon atom at the center and the four Fluorine atoms arranged around it in a symmetrical manner.
How many lone pairs of electrons are present in the Xenon Tetrafluoride Lewis structure?
+The most common representation of the Xenon Tetrafluoride Lewis structure involves two lone pairs of electrons on the Xenon atom.
What are the real-world applications of Xenon Tetrafluoride?
+Xenon Tetrafluoride has several real-world applications, including its use as a fluorinating agent in organic chemistry and in the production of semiconductors and other electronic devices.
In conclusion, the Xenon Tetrafluoride Lewis structure is a fundamental concept in chemistry that represents the arrangement of electrons in the Xenon Tetrafluoride molecule. Understanding this structure is crucial for predicting the chemical properties and reactivity of the molecule, and it has several real-world applications in various fields of chemistry and physics. By exploring the five different ways to represent the Xenon Tetrafluoride Lewis structure, we can gain a deeper understanding of this complex molecule and its unique properties.
Meta Description: Learn about the Xenon Tetrafluoride Lewis structure and its five different representations, and discover its chemical properties, reactivity, and real-world applications.