The Xeo3 Lewis structure, also known as the xenon trioxide molecule, is a fascinating subject in the realm of chemistry. To understand this molecule, we must first delve into the basics of Lewis structures and the properties of xenon. Lewis structures, also known as electron dot diagrams, are a graphical representation of the distribution of electrons in a molecule. They are a crucial tool for understanding the bonding and properties of molecules.
Key Points
- The Xeo3 molecule consists of one xenon atom and three oxygen atoms.
- Xenon is a noble gas, which typically does not react with other elements due to its full outer energy level.
- However, under certain conditions, xenon can form compounds with highly electronegative elements like oxygen.
- The Lewis structure of Xeo3 is crucial for understanding its reactivity and properties.
- Xeo3 is highly reactive and can be dangerous if not handled properly.
Understanding Xenon and Its Compounds
Xenon is a noble gas, situated in the far right column of the periodic table. Noble gases are known for their stability and unreactivity due to their full outer energy level. However, under specific conditions, such as high pressure and temperature, or in the presence of highly electronegative elements, noble gases can form compounds. Xenon, in particular, has been found to form several compounds, including xenon trioxide (Xeo3), which is the focus of this discussion.
Construction of the Xeo3 Lewis Structure
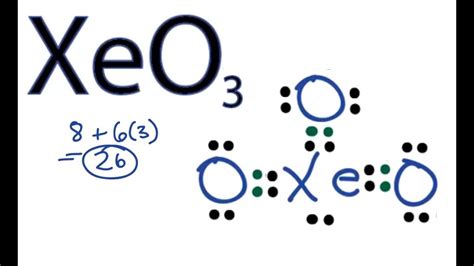
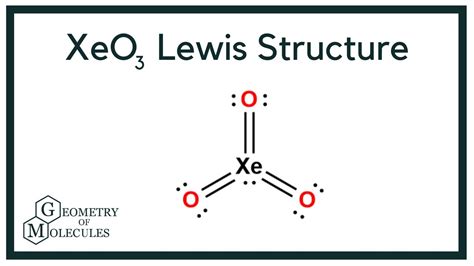
To construct the Lewis structure of Xeo3, we follow a series of steps. First, we determine the total number of valence electrons in the molecule. Xenon has 8 valence electrons, and each oxygen atom has 6 valence electrons. Thus, the total number of valence electrons in Xeo3 is 8 (from xenon) + 3*6 (from three oxygen atoms) = 26 valence electrons.
Next, we draw the skeleton of the molecule, placing the xenon atom in the center and the oxygen atoms around it. Given the highly electronegative nature of oxygen, we then draw single bonds between the xenon and each oxygen atom, which accounts for 6 electrons (2 electrons per bond). This leaves us with 26 - 6 = 20 electrons to distribute.
The remaining electrons are distributed around the oxygen atoms to satisfy the octet rule, which states that each atom should have 8 electrons in its outermost shell. Each oxygen atom already has 2 electrons from the bond with xenon, so we add 6 more electrons to each oxygen (as 3 pairs of electrons) to fulfill the octet rule for the oxygen atoms. This leaves us with no remaining electrons to place on the xenon, which already has its octet fulfilled through the bonds with oxygen and additional electrons placed around it to fulfill the octet rule, making it isoelectronic with the iodate ion.
| Atom | Valence Electrons | Bonding Electrons | Non-bonding Electrons |
|---|---|---|---|
| Xenon | 8 | 6 | 2 (to fulfill octet as part of the molecule) |
| Oxygen (each) | 6 | 2 | 6 (3 pairs) |
Properties and Reactivity of Xeo3
Xenon trioxide is a highly reactive compound due to its instability. It is a strong oxidizing agent and can react violently with organic materials. The compound is also highly toxic and can cause severe damage if inhaled or if it comes into contact with the skin. Due to these properties, handling Xeo3 requires extreme caution and specialized equipment.
Safety Considerations
Given the high reactivity and toxicity of Xeo3, it is crucial to handle this compound with utmost care. This includes wearing appropriate protective gear, working in a well-ventilated area, and following strict protocols for handling and disposing of the compound. The Lewis structure helps in understanding the molecular geometry and reactivity of Xeo3, which is essential for predicting its behavior under different conditions.
Conclusion and Future Directions
The Xeo3 Lewis structure provides valuable insights into the molecular structure and reactivity of xenon trioxide. Understanding the properties and behavior of such compounds is essential for advancing our knowledge of noble gas chemistry and for potential applications in various fields. As research continues to unveil the complexities of noble gas compounds, the importance of detailed structural analysis, such as Lewis structures, becomes increasingly evident.
What is the significance of the Xeo3 Lewis structure in understanding its properties?
+The Xeo3 Lewis structure is crucial for understanding the molecular geometry, bonding, and reactivity of xenon trioxide. It helps in predicting the compound’s behavior and potential interactions with other substances.
Why is xenon trioxide highly reactive?
+Xenon trioxide is highly reactive due to its instability. The compound has a high tendency to release oxygen, making it a strong oxidizing agent. This property, combined with its molecular structure, contributes to its high reactivity.
What safety precautions should be taken when handling Xeo3?
+Handling Xeo3 requires extreme caution. It is essential to wear protective gear, including gloves and a mask, and to work in a well-ventilated area. Following strict protocols for handling and disposing of the compound is also crucial to minimize risks.



